
Ideal Gas Constant
4 min read•december 6, 2021
Using the Ideal Gas Constant in Calculations
For those of you who are taking science classes right now like physics and chemistry, you must be wondering: what's the ideal gas constant? 👀🧪

Image courtesy of Pexels
The ideal gas constant is a number used to predict the behavior of REAL gases. Because gases are challenging to predict, scientists found a way to approximate natural gas behavior. 🌬️
This way connects the different values of
pressure
volume
number of moles, and
temperature.
Important Concepts of Ideal Gases
First, let's discuss some important concepts regarding the nature of ideal gases, which will help you understand how ideal gases react with one another and the "physical" form they occupy. 🔎
Two parts are important to understand when discussing ideal gases:
Ideal gases do not attract or repel each other 🙅
Ideal gases do not take up volume themselves 🙅
There are only elastic collisions between molecules in ideal gases, and no attractions are formed between different molecules. Secondly, ideal gases do not have or take up volume. 🚗
Sounds too good to be true? Well, it is! Ideal gases are used as approximations, but no gas is truly ideal out there. 🤩
Simple Gas Laws
The Ideal Gas Law comprises three rules called the Simple Gas Laws. We can derive these "simple" laws from the ideal gas equation. Below is a summary of the three laws: 📋
1 Boyle’s Law
This law describes an inversely (⬇️) proportional relationship between volume and pressure at a constant temperature. This means that if volume increases, pressure decreases. When volume decreases, pressure increases.
💡 Boyle’s law actually explains the way that we breathe! To learn more, check out this article!
Equation:
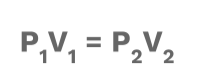
2 Charles's Law
This law describes a directly (⬆️) proportional relationship between temperature (in Kelvins) and volume at a constant pressure. This means that if temperature increases, volume also increases. When temperature decreases, volume will also decrease. 🌡️
Equation:
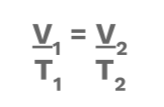
3 Avogadro’s Law
This law describes a directly (⬆️) proportional relationship between volume and amount of gas at a constant pressure and temperature. This means that if volume increases, the amount of gas increases. When volume decreases, the amount of gas decreases. 🛒
Equation:
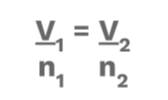
The Equation
The equations above seemed like a lot to remember, right? 😥
Don't worry, there is a much-simplified version, as we mentioned before! Now, it's time to meet the Ideal Gas Law equation, the star of the show! 🌟🥳
Drumroll, please! 🥁
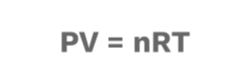
Where…
P = Pressure
V = Volume
n = Number of Moles
R= Ideal Gas Constant
T = Change in Temperature (in Kelvin)
This equation relates the different pressure, volume, R, and temperature changes. The ideal gas law is critical to chemistry and engineering as individuals specializing in either discipline perform necessary calculations involving gases. ✅
Pressure
Pressure is a force exerted on a given area. Pressure is often expressed in units of Pascal but can also be expressed in terms of atm, torr, bar, and the like.
Volume
Volume is the amount of space occupied by an object, often expressed in liters or milliliters.
Wait, what's R, then?
R is the ideal gas constant and is given many different values depending on the different units being used. For example…
R = 8.314 L*kPa/mol*K
R = .0821 L*atm/ mol*K
R = 62.4 L*mmHg/mol*K
When doing calculations, be sure to use the right R value that corresponds to the right units of measurement; otherwise you will end up getting the wrong answer, so make sure to always triple check units! 🚧
Summary of Units Used
Here's a summary of the different units for each property included in the equation!
Property | Variable | Units |
Pressure | P |
|
Volume | V |
|
Moles | n |
|
Temperature | T |
|
Gas Constant | R |
|
Resources
Still unsure whether you've fully grasped this concept or not? 🤷♀️
💡 Check out this article to review all of the material that we have learned in this study guide! For visual and auditory learners, watch this video! Either resource discusses the combination of all of the laws mentioned above… on top of the ideal gas law itself! ⚖️
🤝Connect with other students studying Physics with Hours
Ideal Gas Constant
4 min read•december 6, 2021
Using the Ideal Gas Constant in Calculations
For those of you who are taking science classes right now like physics and chemistry, you must be wondering: what's the ideal gas constant? 👀🧪

Image courtesy of Pexels
The ideal gas constant is a number used to predict the behavior of REAL gases. Because gases are challenging to predict, scientists found a way to approximate natural gas behavior. 🌬️
This way connects the different values of
pressure
volume
number of moles, and
temperature.
Important Concepts of Ideal Gases
First, let's discuss some important concepts regarding the nature of ideal gases, which will help you understand how ideal gases react with one another and the "physical" form they occupy. 🔎
Two parts are important to understand when discussing ideal gases:
Ideal gases do not attract or repel each other 🙅
Ideal gases do not take up volume themselves 🙅
There are only elastic collisions between molecules in ideal gases, and no attractions are formed between different molecules. Secondly, ideal gases do not have or take up volume. 🚗
Sounds too good to be true? Well, it is! Ideal gases are used as approximations, but no gas is truly ideal out there. 🤩
Simple Gas Laws
The Ideal Gas Law comprises three rules called the Simple Gas Laws. We can derive these "simple" laws from the ideal gas equation. Below is a summary of the three laws: 📋
1 Boyle’s Law
This law describes an inversely (⬇️) proportional relationship between volume and pressure at a constant temperature. This means that if volume increases, pressure decreases. When volume decreases, pressure increases.
💡 Boyle’s law actually explains the way that we breathe! To learn more, check out this article!
Equation:
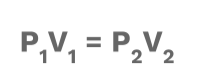
2 Charles's Law
This law describes a directly (⬆️) proportional relationship between temperature (in Kelvins) and volume at a constant pressure. This means that if temperature increases, volume also increases. When temperature decreases, volume will also decrease. 🌡️
Equation:
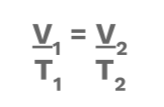
3 Avogadro’s Law
This law describes a directly (⬆️) proportional relationship between volume and amount of gas at a constant pressure and temperature. This means that if volume increases, the amount of gas increases. When volume decreases, the amount of gas decreases. 🛒
Equation:
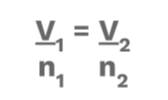
The Equation
The equations above seemed like a lot to remember, right? 😥
Don't worry, there is a much-simplified version, as we mentioned before! Now, it's time to meet the Ideal Gas Law equation, the star of the show! 🌟🥳
Drumroll, please! 🥁
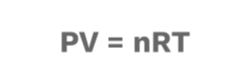
Where…
P = Pressure
V = Volume
n = Number of Moles
R= Ideal Gas Constant
T = Change in Temperature (in Kelvin)
This equation relates the different pressure, volume, R, and temperature changes. The ideal gas law is critical to chemistry and engineering as individuals specializing in either discipline perform necessary calculations involving gases. ✅
Pressure
Pressure is a force exerted on a given area. Pressure is often expressed in units of Pascal but can also be expressed in terms of atm, torr, bar, and the like.
Volume
Volume is the amount of space occupied by an object, often expressed in liters or milliliters.
Wait, what's R, then?
R is the ideal gas constant and is given many different values depending on the different units being used. For example…
R = 8.314 L*kPa/mol*K
R = .0821 L*atm/ mol*K
R = 62.4 L*mmHg/mol*K
When doing calculations, be sure to use the right R value that corresponds to the right units of measurement; otherwise you will end up getting the wrong answer, so make sure to always triple check units! 🚧
Summary of Units Used
Here's a summary of the different units for each property included in the equation!
Property | Variable | Units |
Pressure | P |
|
Volume | V |
|
Moles | n |
|
Temperature | T |
|
Gas Constant | R |
|
Resources
Still unsure whether you've fully grasped this concept or not? 🤷♀️
💡 Check out this article to review all of the material that we have learned in this study guide! For visual and auditory learners, watch this video! Either resource discusses the combination of all of the laws mentioned above… on top of the ideal gas law itself! ⚖️
🤝Connect with other students studying Physics with Hours

Resources
© 2024 Fiveable Inc. All rights reserved.
AP® and SAT® are trademarks registered by the College Board, which is not affiliated with, and does not endorse this website.