
7.14 Free Energy of Dissolution
4 min read•january 8, 2023
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
The final section of Unit 7 brings together two topics: solubility and thermodynamics, which in AP Chemistry can be defined as the study of energy transfers during chemical reactions. Note that we used the word energy and not a word like “temperature” or “heat.” This is because heat is only one component of thermodynamics.
We discussed heat in Unit 6 when discussing enthalpy changes to describe a reaction as either exothermic or endothermic, meaning heat-releasing or heat-absorbing. However, there are two more thermodynamic measures that will be important for this unit and during Unit 9, which goes into much more detail about them: entropy (notated as S) and Gibbs Free Energy (notated as G). While we will wait until Unit 9 to dive deep into these concepts, in this guide, we will briefly discuss these measures and how they relate to the dissolution of soluble and sparingly soluble (what we would usually call “insoluble”) compounds.
Brief Introduction to Entropy, Gibbs Free Energy, and Thermodynamic Favorability
Before we start learning about actually dissolving substances, let’s do a quick crash course in thermodynamics so we better understand what the underlying forces of energy are when we deal with dissolution.
Entropy
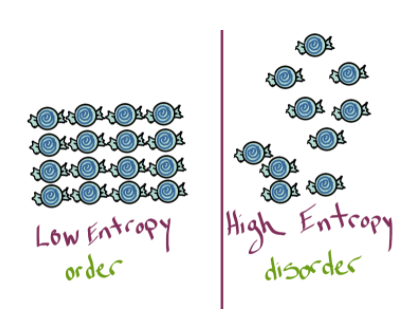
The first important concept is that of entropy. In layman’s terms, entropy describes the amount of “disorder” in a system. Entropy is also described as the number of possible arrangements in a system. Essentially, the more spread out and chaotic the system is, the higher the entropy.
We care about entropy because it describes energy flows when a system becomes more ordered. Let’s think about a less chemical example first. Suppose you have a bedroom. It is pretty easy to make your bedroom messy — rip the sheets off the bed, throw clothes on the floor, pull down posters, and go wild (sorry, parents). In this instance, you have increased your room's entropy by making it more chaotic. However, it takes a lot of energy to pick up the mess you made and return your room to a more “ordered” state. This shows the idea that systems naturally tend towards entropy, and it takes energy to keep them ordered, such as cleaning your room.
The most common chemical example of entropy changes is in melting and freezing. The melting and freezing of a substance can be described as follows (in this example we use water, but it applies to any other substance).
H2O (s) ⇌ H2O (l) ⇌ H2O (g)
We have solid water (ice) melting into liquid water and then evaporating into water vapor. In any one of these instances, entropy is increasing. In the opposite direction, entropy is decreasing. Essentially solids are the most ordered, followed by liquids, and then gases.
Gibbs Free Energy and Thermodynamic Favorability
So far, we have learned about two measures of thermodynamics: enthalpy and entropy. Gibbs Free Energy combines these two values to describe the thermodynamic favorability for a reaction. The formula for Gibbs Free Energy change is as follows:
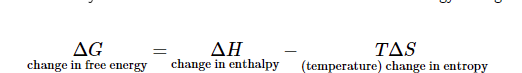
When delta G is negative, we describe the reaction as “spontaneous” or “thermodynamically favorable.” Conversely, when delta G is positive, the reaction is “nonspontaneous” or “thermodynamically unfavorable.” These words describe the relationship between enthalpy and entropy changes and how they relate to how far forward a reaction will go.
Although these concepts will be examined in greater detail in Unit 9, delta G has an intimate relationship with K, the equilibrium constant. A spontaneous reaction will have a K greater than 1 and vice versa.
Gibbs Free Energy and Dissolving Substances
We describe enthalpy and entropy changes when dissolving in the following table:

Note that enthalpy changes refer to energy being released or absorbed to break attractions. In contrast, entropy changes refer to order or disorder.
It is important to remember that soluble and insoluble compounds exist as crystal lattices when in their solid state. Therefore, in order for a substance to dissolve, the solute-solute attractions that form the lattice must be broken down. Similarly, the intermolecular forces between the solvent, such as hydrogen bonds between water molecules, must be broken. Breaking these bonds takes energy, and forming the new attractions between ions and water molecules releases energy.
Gibbs Free Energy change helps guide whether or not a dissolution will be spontaneous at standard conditions. Remember from before that ΔG = ΔH - TΔS. Therefore, both enthalpy and entropy play into the spontaneity of dissolution. If ΔG is positive, our Ksp will be very small.
Key Terms to Review (18)
Crystal Lattices
: A crystal lattice is a repeating pattern of atoms, ions or molecules in a crystalline substance, arranged in a highly ordered, symmetrical manner.Dissolution
: Dissolution is the process by which a solute in gaseous, liquid, or solid phase dissolves in a solvent to form a solution.Endothermic Reaction
: An endothermic reaction is one that absorbs heat from its surroundings. In this process, more heat goes into breaking bonds in reactants than gets released when new bonds form in products.Enthalpy
: Enthalpy is a measure of total energy in a thermodynamic system. It includes internal energy which can be used for work and volume expansion against an external pressure.Entropy
: Entropy refers to the measure of disorder or randomness in a system. In chemistry, higher entropy means higher disorder and less predictability.Equilibrium Constant (K)
: The equilibrium constant (K) is a measure of the ratio of concentrations at equilibrium for products over reactants, each raised to their stoichiometric coefficients in the balanced equation.Exothermic Reaction
: An exothermic reaction is a chemical reaction that releases energy by light or heat. It is the opposite of an endothermic reaction.Freezing
: Freezing is the process in which a substance changes from a liquid to a solid state, usually as a result of temperature decrease.Gibbs Free Energy
: Gibbs Free Energy (G) is a thermodynamic potential that measures the maximum reversible work that a system can perform at constant temperature and pressure.Hydrogen bonds
: A type of attractive intermolecular force that occurs when a hydrogen atom bonded to a highly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons.Intermolecular Forces
: These are the forces that occur between molecules. They're weaker than intramolecular forces but still crucial for determining properties like boiling and melting points.Melting
: Melting is the process where a solid turns into a liquid due to an increase in temperature or pressure.Nonspontaneous Reaction
: A nonspontaneous reaction is a chemical reaction that does not occur naturally without the input of energy.Solubility
: Solubility is the maximum amount of a solute that can be dissolved in a solvent at a given temperature.Solute-Solute Attractions
: These are the forces of attraction between particles (molecules or ions) of a solute in a solution. They must be overcome for the solute to dissolve.Sparingly Soluble Compounds
: Sparingly soluble compounds are those that dissolve only to a small extent in a solvent. They form solutions with low concentrations of solute particles.Spontaneous Reaction
: A spontaneous reaction is one that can occur without any external input once it has started. These reactions are thermodynamically favorable and often release energy in some form.Thermodynamics
: Thermodynamics is the study of energy and its transformations from one form to another.7.14 Free Energy of Dissolution
4 min read•january 8, 2023
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
The final section of Unit 7 brings together two topics: solubility and thermodynamics, which in AP Chemistry can be defined as the study of energy transfers during chemical reactions. Note that we used the word energy and not a word like “temperature” or “heat.” This is because heat is only one component of thermodynamics.
We discussed heat in Unit 6 when discussing enthalpy changes to describe a reaction as either exothermic or endothermic, meaning heat-releasing or heat-absorbing. However, there are two more thermodynamic measures that will be important for this unit and during Unit 9, which goes into much more detail about them: entropy (notated as S) and Gibbs Free Energy (notated as G). While we will wait until Unit 9 to dive deep into these concepts, in this guide, we will briefly discuss these measures and how they relate to the dissolution of soluble and sparingly soluble (what we would usually call “insoluble”) compounds.
Brief Introduction to Entropy, Gibbs Free Energy, and Thermodynamic Favorability
Before we start learning about actually dissolving substances, let’s do a quick crash course in thermodynamics so we better understand what the underlying forces of energy are when we deal with dissolution.
Entropy
The first important concept is that of entropy. In layman’s terms, entropy describes the amount of “disorder” in a system. Entropy is also described as the number of possible arrangements in a system. Essentially, the more spread out and chaotic the system is, the higher the entropy.
We care about entropy because it describes energy flows when a system becomes more ordered. Let’s think about a less chemical example first. Suppose you have a bedroom. It is pretty easy to make your bedroom messy — rip the sheets off the bed, throw clothes on the floor, pull down posters, and go wild (sorry, parents). In this instance, you have increased your room's entropy by making it more chaotic. However, it takes a lot of energy to pick up the mess you made and return your room to a more “ordered” state. This shows the idea that systems naturally tend towards entropy, and it takes energy to keep them ordered, such as cleaning your room.
The most common chemical example of entropy changes is in melting and freezing. The melting and freezing of a substance can be described as follows (in this example we use water, but it applies to any other substance).
H2O (s) ⇌ H2O (l) ⇌ H2O (g)
We have solid water (ice) melting into liquid water and then evaporating into water vapor. In any one of these instances, entropy is increasing. In the opposite direction, entropy is decreasing. Essentially solids are the most ordered, followed by liquids, and then gases.

Gibbs Free Energy and Thermodynamic Favorability
So far, we have learned about two measures of thermodynamics: enthalpy and entropy. Gibbs Free Energy combines these two values to describe the thermodynamic favorability for a reaction. The formula for Gibbs Free Energy change is as follows:
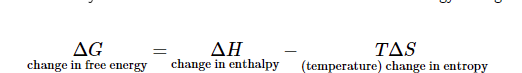
When delta G is negative, we describe the reaction as “spontaneous” or “thermodynamically favorable.” Conversely, when delta G is positive, the reaction is “nonspontaneous” or “thermodynamically unfavorable.” These words describe the relationship between enthalpy and entropy changes and how they relate to how far forward a reaction will go.
Although these concepts will be examined in greater detail in Unit 9, delta G has an intimate relationship with K, the equilibrium constant. A spontaneous reaction will have a K greater than 1 and vice versa.
Gibbs Free Energy and Dissolving Substances
We describe enthalpy and entropy changes when dissolving in the following table:

Note that enthalpy changes refer to energy being released or absorbed to break attractions. In contrast, entropy changes refer to order or disorder.
It is important to remember that soluble and insoluble compounds exist as crystal lattices when in their solid state. Therefore, in order for a substance to dissolve, the solute-solute attractions that form the lattice must be broken down. Similarly, the intermolecular forces between the solvent, such as hydrogen bonds between water molecules, must be broken. Breaking these bonds takes energy, and forming the new attractions between ions and water molecules releases energy.
Gibbs Free Energy change helps guide whether or not a dissolution will be spontaneous at standard conditions. Remember from before that ΔG = ΔH - TΔS. Therefore, both enthalpy and entropy play into the spontaneity of dissolution. If ΔG is positive, our Ksp will be very small.
Key Terms to Review (18)
Crystal Lattices
: A crystal lattice is a repeating pattern of atoms, ions or molecules in a crystalline substance, arranged in a highly ordered, symmetrical manner.Dissolution
: Dissolution is the process by which a solute in gaseous, liquid, or solid phase dissolves in a solvent to form a solution.Endothermic Reaction
: An endothermic reaction is one that absorbs heat from its surroundings. In this process, more heat goes into breaking bonds in reactants than gets released when new bonds form in products.Enthalpy
: Enthalpy is a measure of total energy in a thermodynamic system. It includes internal energy which can be used for work and volume expansion against an external pressure.Entropy
: Entropy refers to the measure of disorder or randomness in a system. In chemistry, higher entropy means higher disorder and less predictability.Equilibrium Constant (K)
: The equilibrium constant (K) is a measure of the ratio of concentrations at equilibrium for products over reactants, each raised to their stoichiometric coefficients in the balanced equation.Exothermic Reaction
: An exothermic reaction is a chemical reaction that releases energy by light or heat. It is the opposite of an endothermic reaction.Freezing
: Freezing is the process in which a substance changes from a liquid to a solid state, usually as a result of temperature decrease.Gibbs Free Energy
: Gibbs Free Energy (G) is a thermodynamic potential that measures the maximum reversible work that a system can perform at constant temperature and pressure.Hydrogen bonds
: A type of attractive intermolecular force that occurs when a hydrogen atom bonded to a highly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons.Intermolecular Forces
: These are the forces that occur between molecules. They're weaker than intramolecular forces but still crucial for determining properties like boiling and melting points.Melting
: Melting is the process where a solid turns into a liquid due to an increase in temperature or pressure.Nonspontaneous Reaction
: A nonspontaneous reaction is a chemical reaction that does not occur naturally without the input of energy.Solubility
: Solubility is the maximum amount of a solute that can be dissolved in a solvent at a given temperature.Solute-Solute Attractions
: These are the forces of attraction between particles (molecules or ions) of a solute in a solution. They must be overcome for the solute to dissolve.Sparingly Soluble Compounds
: Sparingly soluble compounds are those that dissolve only to a small extent in a solvent. They form solutions with low concentrations of solute particles.Spontaneous Reaction
: A spontaneous reaction is one that can occur without any external input once it has started. These reactions are thermodynamically favorable and often release energy in some form.Thermodynamics
: Thermodynamics is the study of energy and its transformations from one form to another.
Resources
© 2024 Fiveable Inc. All rights reserved.
AP® and SAT® are trademarks registered by the College Board, which is not affiliated with, and does not endorse this website.